
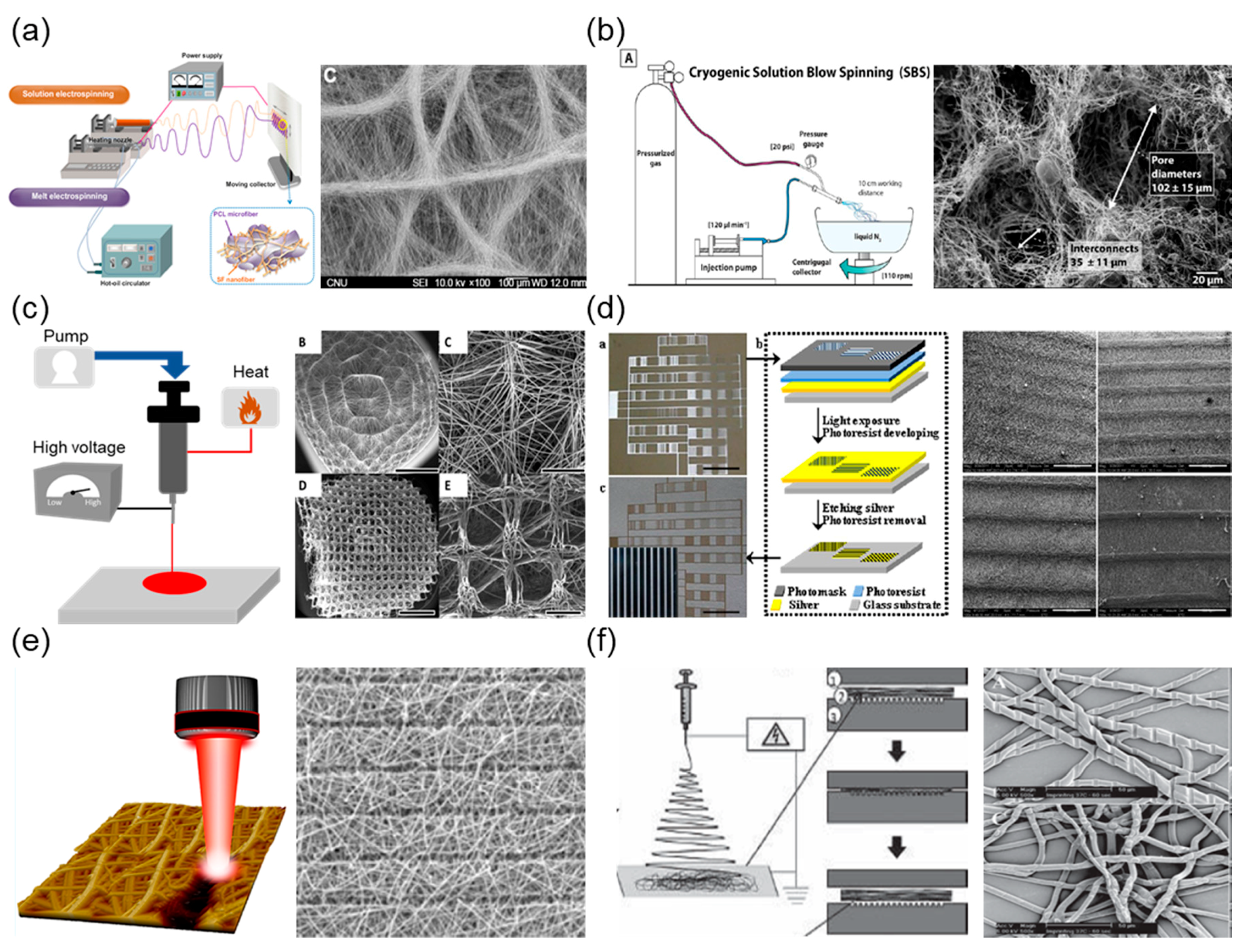
This includes the development of FRESH and embedded 3D printing within the bioprinting field and the rapid growth in adoption, as well as the advantages of FRESH printing for biofabrication and the new research results this has enabled. In this Perspective, we discuss the challenges of 3D printing soft and liquid-like bioinks and the emergence for FRESH and related embedded printing techniques as a solution. Freeform Reversible Embedding of Suspended Hydrogels (FRESH) is an embedded printing approach that solves this problem by extruding bioinks within a yield-stress support bath that holds the bioinks in place until cured. Additive manufacturing techniques, such as 3D bioprinting, have the potential to build biological material with unprecedented spatial control however, printing soft biological materials in air often results in poor fidelity.
#3d printing tissue engineering scaffold advantage how to#
This effort has resulted in the first entirely 3D-printed organ on a chip – a heart on a chip – with integrated soft strain sensors.In tissue engineering, an unresolved challenge is how to build complex 3D scaffolds in order to recreate the structure and function of human tissues and organs. The Wyss team is also investigating the use of 3D bioprinting to fabricate new versions of the Institute’s organs on chips devices, which makes their manufacturing process more automated and enables development of increasingly complex microphysiological devices. This innovative bioprinting approach can be modified to create various vascularized 3D tissues for regenerative medicine and drug testing endeavors. In a proof-of-principle study, one centimeter thick bioprinted tissue constructs containing human bone marrow MSCs surrounded by connective tissue and supported by an artificial endothelium-lined vasculature, allowed the circulation of bone growth factors and, subsequently, the induction of bone development. The resulting soft tissue structure can be immediately perfused with nutrients as well as growth and differentiation factors via a single inlet and outlet on opposite ends of the chip that connect to the vascular channel to ensure survival and maturation of the cells. Credit: Lewis Lab, Wyss Institute at Harvard University The structure was fabricated using a novel 3D bioprinting strategy invented by Jennifer Lewis and her team at the Wyss Institute and Harvard SEAS. Confocal microscopy image showing a cross-section of a 3D-printed, 1-centimeter-thick vascularized tissue construct showing stem cell differentiation towards development of bone cells, following one month of active perfusion of fluids, nutrients, and cell growth factors.

After printing, a liquid composed of fibroblasts and extracellular matrix is used to fill open regions within the construct, adding a connective tissue component that cross-links and further stabilizes the entire structure.
Inside this mold, a grid of larger vascular channels containing living endothelial cells in silicone ink is printed, into which a self-supporting ink containing living mesenchymal stem cells (MSCs) is layered in a separate print job. The method uses a customizable, printed silicone mold to house and plumb the printed tissue on a chip. Multidisciplinary research at the Wyss Institute has led to the development of a multi-material 3D bioprinting method that generates vascularized tissues composed of living human cells that are nearly ten-fold thicker than previously engineered tissues and that can sustain their architecture and function for upwards of six weeks. Their work sets the stage for advancement of tissue replacement and tissue engineering techniques. Play In this video, the Wyss Institute and Harvard SEAS team uses a customizable 3D bioprinting method to build a thick vascularized tissue structure comprising human stem cells, collective matrix, and blood vessel endothelial cells.


 0 kommentar(er)
0 kommentar(er)
